전한울
2024년 10월 1일
- -Mature mammalian neurons lose ability to regenerate CNS axons
-Contrast between immature and mature neurons' regenerative capacity
-Neuronal maturation transitions from growth to information transmission
-Loss of regenerative ability coincides with synapse formation
-Understanding maturation processes may identify targets for repair
Abstract
Mammalian neurons lose the ability to regenerate their central nervous system axons as they mature during embryonic or early postnatal development. Neuronal maturation requires a transformation from a situation in which neuronal components grow and assemble to one in which these components are fixed and involved in the machinery for effective information transmission and computation. To regenerate after injury, neurons need to overcome this fixed state to reactivate their growth programme. A variety of intracellular processes involved in initiating or sustaining neuronal maturation, including the regulation of gene expression, cytoskeletal restructuring and shifts in intracellular trafficking, have been shown to prevent axon regeneration. Understanding these processes will contribute to the identification of targets to promote repair after injury or disease.

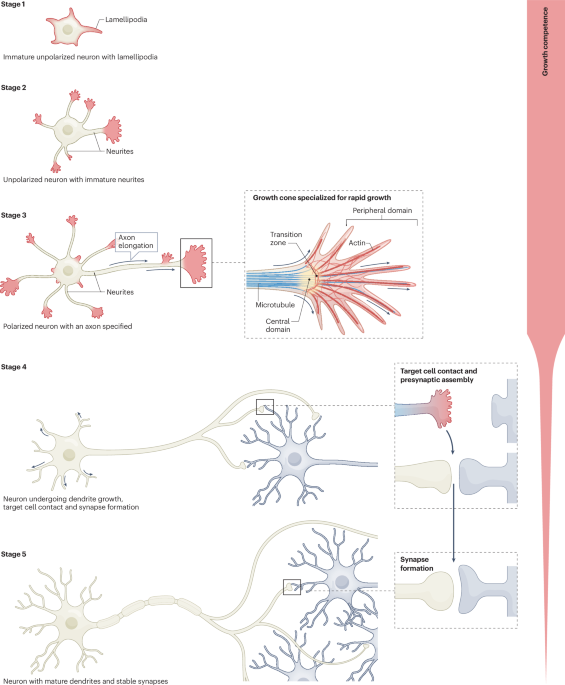
In unpolarized neurons (stage 2), the transport of cargo into neurites is unrestricted31. Specifically, there is a vectorial cytoplasmatic flow in the growing axon that pushes organelles, exocytic vesicles and dendritic membrane proteins into the axon110. In neurons that have polarized and have rapidly growing axons (stage 3), growth-associated cargo is quickly transported to the growth cone and/or distal axon to enable outgrowth. This cargo includes integrins and neural cell adhesion molecule L1 (L1CAM), which interact with the extracellular matrix and cell surfaces to drive axon growth122,123,134. Mitochondria are also rapidly transported in immature neurons to supply adequate energy for axon outgrowth220. As the neuron matures, transport within the axon and dendrites becomes highly polarized due to the orientation of microtubules and the presence of specific motor proteins. In axons, which have almost exclusively plus-end-out microtubules, KIF5 and dynein–dynactin drive anterograde and retrograde transport, respectively221. By contrast, in dendrites, dynein–dynactin directs transport to the proximal dendrites and soma through bidirectional runs of microtubules. Growth-associated protein delivery to the growth cone also declines with maturity, contributing to deceleration of axon outgrowth and loss of axon growth competence111. Mitochondrial transport to the axon becomes stalled, partly owing to increased axonal expression of syntaphilin, a mitochondrial docking protein that decelerates mitochondrial transport along the axon63. Many mechanisms related to the axon initial segment control transport into axons in mature neurons, including the exclusion of dendrite-directed endosomes at the pre-axonal exclusion zone222. To provide an example of the complexity of polarized transport, a mechanism for the exclusion of integrins from mature axons is shown. Recycling endosomes containing Ras-related protein 11 (RAB11) and ADP-ribosylation factor 6 (ARF6) carry out most transport of integrins to immature axons47. However, in mature neurons, the guanine exchange factor EFA6 is upregulated at the axon initial segment. This restricts integrin from axons by activating RAB11-linked ARF6, which causes the transport adaptor c-Jun NH2-terminal kinase (JNK)-interacting protein 4 (JIP4) to attach the RAB11 endosomes to dynein for retrograde transport111. In mature neurons, most transport to dendrites is carried out by RAB8 endosomes, which contain postsynaptic molecules223, while RAB3 endosomes or vesicles carry much of the machinery required for neurotransmission to the axon tip224.